Brain Cancer
Welcome to Dr Khurana’s Brain Cancer page.
Images shown here are with the permission of Dr Khurana’s patients, for educational purposes.
Please also visit the Awake Craniotomy pages for details of Dr Khurana’s experience and expertise in awake brain microsurgery.
For a YouTube video of Dr Khurana’s brain tumour surgery, CLICK HERE
Click any of the images below for a larger view.
Brain cancers are aggressive tumours that grow in and invade the brain. They can either grow from brain tissue cells themselves (“primary” brain tumours) or grow from tissue cells that have migrated/spread to the brain from other more remote locations (“secondary” or “metastatic” deposits).
The majority of primary brain tumours occur spontaneously (i.e., for no known reason); some occur as a result of exposure to brain radiation (i.e., several years after brain radiation therapy; a small but known risk). Rarely, some primary brain tumours are associated with family genetics – e.g., Li-Fraumeni syndrome (a rare, inherited condition that dramatically increases the risk of many types of cancer). There is ongoing debate regarding a relationship between substantial long-term mobile phone radiation exposure and the occurrence of primary brain tumours.
- Examples of primary brain tumour types are “gliomas”, “astrocytomas”, “oligodendrogliomas”, “glioblastoma” and “gliosarcoma”;
- Examples of metastases that can seed the brain are melanoma, breast, lung, renal (kidney), colorectal, uterine and ovarian cancers.
Currently, for patients with brain cancer, the key to the quantity and quality of life is the surgeon and the type of surgery.
In the most aggressive forms of primary brain cancer, anaplastic astrocytoma (WHO grade III) and glioblastoma multiforme (WHO grade IV), certain genetic biomarkers have been identified that are associated with better or worse patient survival.
- Mutations of tumour suppressor genes PTEN (“phosphatase and tensin homolog” gene) and/or TP53 (“tumor protein p53” gene) WORSEN the prognosis.
- Presence of UNmutated (i.e., “wild-type”) IDH1 (“isocitrate dehydrogenase 1”) gene WORSENS the prognosis.
- Methylation of the promoter of MDMT (“methylguanine-DNA methyltransferase”) gene IMPROVES the prognosis.
- Overactivity of the EGFR (“epidermal growth factor”) gene pathway (i.e., EGFR gene amplification and overexpression) is associated with RESISTANCE to radiotherapy and chemotherapy, which WORSENS prognosis.
- The presence of 1p/19q co-deletion is associated with enhanced sensitivity to radiation and chemotherapy, which IMPROVES prognosis in certain gliomas (especially those with an oligodendroglial component).
IN GENERAL, IN GLIOBLASTOMA MULTIFORME, THE BEST PROGNOSIS WOULD PROBABLY BE IN A YOUNGER PATIENT WHO HAS HAD AN AGGRESSIVE (NEAR-TOTAL) SURGICAL DEBULKING, AND IS UNDERGOING POST-OPERATIVE COMBINED CHEMO AND RADIATION TREATMENT, AND WHOSE BRAIN CANCER CELLS HAVE BOTH METHYLATED MDMT AND MUTATED IDH1.
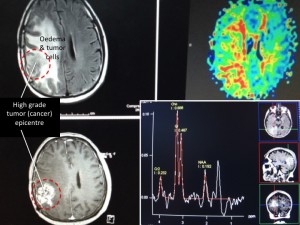
Panel above: A high-grade brain tumor (glioblastoma multiforme, GBM) being imaged on an MRI’s FLAIR (upper left), perfusion (upper right), T1 contrast (lower left) and spectroscopic (lower right) sequences.
Click any of the images for a larger view.
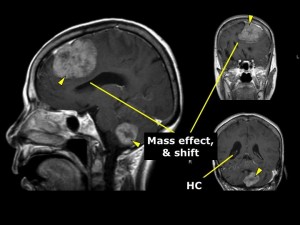
Panel above: This older lady presented with symptoms and signs of raised intracranial pressure, in addition to personality changes and unsteady gait. Her multiple tumours were removed in sequence; they were breast cancer metastases.
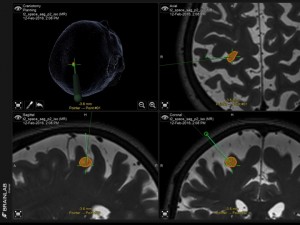
Panel above: This lady had a past diagnosis of uterine cancer. This lesion (volume-mapped in orange) was found in her brain’s main movement area (motor strip), and it was removed uneventfully and minimally invasively by awake craniotomy microsurgery while we tested her neurologically in real-time. The lesion was a uterine cancer met.
Panel above: This man was previously treated for colorectal cancer, but unfortunately this mass developed near his brainstem. It was debulked uneventfully after posterior fossa craniectomy (owing to high pressure in his brain from tumour-related oedema/cerebellar swelling).
Click any of the images for a larger view.
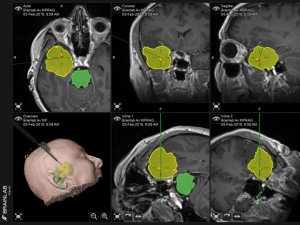
Panel above: This lady had a large glioblastoma multiforme (GBM, highest grade brain cancer; yellow mapped region) removed uneventfully by temporal lobectomy.
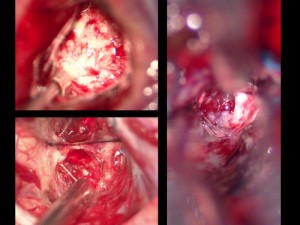
Panel above: Intraoperative views through the operating microscope of brain tumours:
- Low grade glioma (less aggressive primary brain tumor; upper left) – World Health Organisation (WHO) grades 1 and 2 (of 4).
- High grade glioma (more aggressive primary brain tumor, e.g., anaplastic astrocytoma/AA/WHO grade 3, or glioblastoma multiforme/GBM/WHO grade 4; lower left).
- Metastatic cancer (aggressive secondary brain tumor that arose somewhere else in the body but migrated to and invaded the brain; right panel).
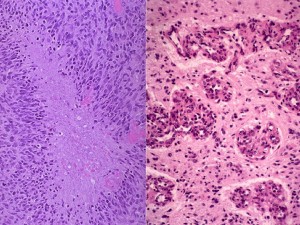
Panel above: What a neuropathologist sees down their own microscope after appropriate staining of the tissue samples we send them from the operating theatre. The above panels show key features of the most aggressive form of primary brain cancer, a glioblastoma multiforme (GBM).
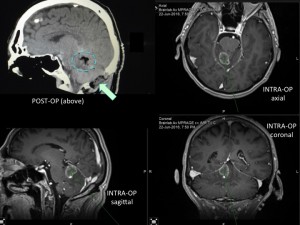
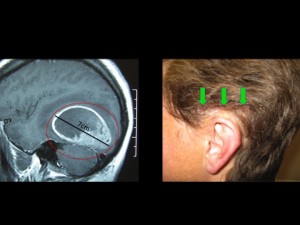
Panel above: This lady presented with a very large primary brain cancer, a GBM. Her brain was swollen, and without surgery she would have lived a few weeks at most. I operated on her twice over a period of eight years, both surgeries were undertaken while she was awake, comfortable and interactive with us. Her survival from a GBM (eight years) was far more than the average survival for this tumour (1.5-2 years). Note the incision, which was photographed one week after her first surgery (green arrows). I use minimally invasive microsurgical techniques for my awakes. I encourage my patients to Never Give Up.